Abstract
Background : ALXN1210 is a rationally designed humanized monoclonal antibody to complement component 5 (C5), engineered to achieve extended half-life and duration of complement inhibition while retaining the proven efficacy and safety of eculizumab.
Objective : ALXN1210-PNH-103 (103) (NCT02598583) and ALXN1210-PNH-201 (201) (NCT02605993) are phase 1/2 multicenter, open-label studies evaluating the safety, tolerability, and efficacy of multiple doses and dosing interval regimens of ALXN1210 in patients with PNH aged >18 years who were naive to complement inhibitor therapy. Interim data from 103 provided preliminary evidence that ALXN1210 provides rapid and sustained blockage of complement-mediated hemolysis.
Methods : In 103, 6 patients in Cohort 1 (C1) received a 400 or 600 mg intravenous (IV) induction dose followed by a 900 mg maintenance dose every 4 weeks (q4w). Seven patients in Cohort 2 (C2) received 600 and 900 mg induction doses followed by 1800 mg maintenance doses q4w. In 201, 6 patients in C1 received an induction dose of 1400 mg on day 1, 1000 mg on days 15 and 29 and q4w thereafter. Six patients in C2 received an induction dose of 2000 mg on day 1, 1600 mg on days 22 and 43 and q6w thereafter. Seven patients in Cohort 3 (C3) received induction doses of 1600 mg on days 1 and 15, 2400 mg on day 29 and q8w thereafter. Seven patients in Cohort 4 (C4) received a 3000 mg induction dose on day 1, 5400 mg on day 29 and q12w thereafter. All cohort doses employed in 201 were calculated to have plasma trough exposures similar to 103 /C1. The primary efficacy endpoint was change in mean plasma lactate dehydrogenase (LDH) levels at the primary completion date of 24w (103) or 36w (201). Other protocol-specified endpoints included free hemoglobin (Hb), FACIT-Fatigue score, and adverse events (AEs). Post hoc efficacy endpoints of interest included proportion of patients reaching LDH normalization and incidence of breakthrough hemolysis (days 29-253). Descriptive statistics were employed for continuous variables; a priori comparisons between cohorts were not conducted.
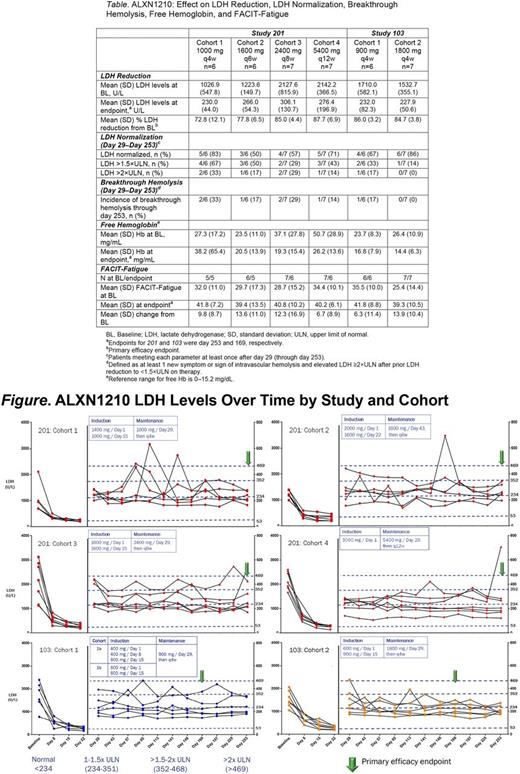
Results : Of 50 patients screened, 39 were enrolled (13 in 103 and 26 in 201). Of 11 screen failures, 7 were due to low LDH. All patients were evaluable through the primary completion date. Most patients in 103 were Asian (92%) and female (54%); the majority of patients in 201 were Caucasian (58%) and male (77%). Rapid and sustained reduction of LDH levels from BL was observed across all treatment groups in both studies, ranging from 73% (201 /C1) to 88% (201 /C4). LDH normalization was experienced to a greater extent in patients receiving ALXN1210 1800 mg q4w (103 /C2), relative to other patients in both studies (Table ; Figure). Similarly, no patients in 103 /C2 experienced breakthrough hemolysis, while breakthrough hemolysis did occur in all other study cohorts (Table). Following treatment, mean free Hb decreased into the normal range in 103 /C2 but remained elevated in all other cohorts (Table). Mean FACIT-Fatigue scores increased in all cohorts (by 6.3-13.9 vs BL; Table).
No deaths, drug discontinuations, or AEs leading to withdrawals occurred in either study. The most frequently reported treatment-related AE was headache, occurring most commonly at initiation of treatment (6/39 [15%]). Two patients in 201 experienced meningococcal infections presenting as sepsis. Patient 1 (18-year-old male, C3), whose blood culture grew penicillin-resistant serogroup Y, and Patient 2 (28-year-old male, C2), whose blood culture grew serogroup Y/W135, were vaccinated with both ACWY and B vaccines. Patient 2 received penicillin prophylaxis but the meningococcal isolate was intermediate in sensitivity to penicillin. Both patients completely recovered following IV ceftriaxone and continued in the study receiving ALXN1210.
Conclusions : In PNH patients naive to complement inhibitor therapy, ALXN1210 treatment resulted in rapid and sustained reduction in plasma LDH levels and improved FACIT-Fatigue score at dose intervals up to q12w. In these studies, higher ALXN1210 plasma trough exposure (in the 1800 mg q4w cohort) was associated with a greater proportion of patients reaching plasma LDH normalization, reductions in free Hb, and lack of breakthrough hemolysis relative to all other cohorts. ALXN1210 exhibited an acceptable safety profile. These findings and subsequent PK/PD analysis informed the dosing strategy for the ALXN1210 phase 3 trials.
Roeth: Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Rottinghaus: Alexion Pharmaceuticals, Inc.: Employment, Equity Ownership. Hill: Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Schrezenmeier: Alexion Pharmaceuticals, Inc.: Honoraria, Other: Research Funding to University of Ulm . Terriou: Alexion Pharmaceuticals, Inc.: Other: Travel support. Wells: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Szer: Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding. Aguzzi: Alexion Pharmaceuticals, Inc.: Employment, Equity Ownership. Bachman: Alexion Pharmaceuticals, Inc.: Employment, Equity Ownership. Damokosh: Alexion Pharmaceuticals, Inc.: Employment, Equity Ownership. Shafner: Alexion Pharmaceuticals, Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.